Abstract
Background
Prognosis is poor for patients (pts) with relapsed/refractory multiple myeloma (RRMM; median overall survival: <1 year), indicating a clear need to identify agents with novel mechanisms of action. B-cell maturation antigen (BCMA) has recently emerged as a novel treatment target for MM due to its highly selective expression in plasma cells. TNB-383B, a BCMA x CD3 T-cell engaging bispecific antibody, was designed to overcome the toxicity limitations of existing BCMA therapies and has demonstrated promising results in an ongoing first-in-human phase 1 study in pts with RRMM (Rodriguez C, et al. Blood 2020;136[suppl 1]:43-44). Herein, we report the updated efficacy and safety outcomes of this phase 1 study.
Methods
This multicenter, phase 1, open-label, dose-escalation/expansion study (NCT03933735) enrolled pts with RRMM (≥3 prior lines of therapy that included a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 monoclonal antibody), estimated glomerular filtration rate ≥30 mL/min as calculated by the Modification of Diet in Renal Disease formula, and ECOG performance status ≤2. Prior BCMA-targeted therapy is prohibited. Primary objectives include safety/tolerability, pharmacokinetics (PK), and determination of recommended phase 2 dose (RP2D); secondary objectives include assessment of clinical activity (per IMWG criteria 2016). TNB-383B is administered intravenously over 1-2 hours every 3 weeks (Q3W) with the first dose administered inpatient. The study uses a 3+3 design with backfilling for dose and permits intrapatient dose escalation to highest safe dose. Dose expansion was initiated on selection of RP2D. Pts are treated until progression, unacceptable toxicity, or other discontinuation criteria are met. The efficacy-evaluable population includes all pts who received at least 1 dose of TNB-383B and have at least 1 postdose assessment. The safety population includes all pts who received at least 1 dose of study drug; adverse events (AEs) are graded according to NCI CTCAE v5.0.
Results
As of 10 May 2021, 103 pts (dose escalation, n=73; dose expansion, n=30) have been treated with TNB-383B (0.025-120 mg). The RP2D of 60 mg Q3W was selected on the basis of tolerability, safety, PK, and clinical activity. Pt demographics and baseline characteristics are summarized in Table 1. Three dose-limiting toxicities were reported in dose escalation (platelet count decreased: grade [Gr] 4, 60 mg; cytokine release syndrome [CRS]: Gr 3, 90 mg and 120 mg); none were reported as serious.
Treatment-related AEs (TRAEs) were reported in 79 (77%) pts, with Gr ≥3 and serious AEs occurring in 33 (32%) and 23 (22%) pts, respectively. The most common TRAEs (Table 2) include CRS (n=54, 52%), neutropenia (n=18, 17%), and fatigue (n=14, 14%). At the RP2D (n=39), the Gr ≥3 CRS rate was 3% (n=1). Onset of CRS typically occurred on the same or next day following the first dose and all pts recovered using tocilizumab or standard supportive care measures. Treatment-emergent AEs (TEAEs) of infusion-related reactions were reported in 8 (8%) pts and infections occurred in 29 (28%) pts; pneumonia (n=5, 5%) and upper respiratory tract infection (n=4, 4%) were the most common. Five deaths from TEAEs were reported; all were unrelated to study drug. Forty-two (40%) pts discontinued treatment due to disease progression.
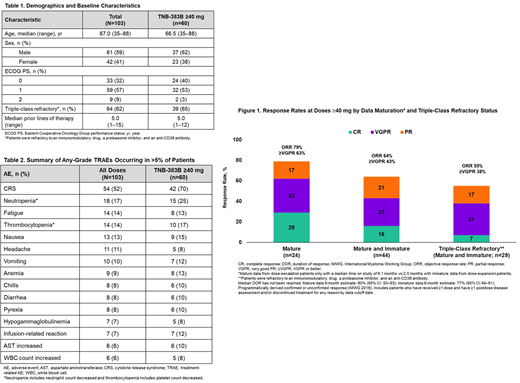
In the dose-escalation cohorts of ≥40 mg Q3W (n=24), the objective response rate (ORR) was 79% (19/24), with a very good partial response or better (≥VGPR) rate of 63% (15/24), and a complete response (CR) rate of 29% (7/24) at the data cutoff date; these pts have the longest follow-up (ie, mature data) with median time on study of 6.1 months (Figure 1). At doses ≥40 mg in the combined dose-escalation and -expansion cohorts (n=44), the observed ORR, ≥VGPR, and CR rates were 64% (28/44), 43% (19/44), and 16% (7/44), respectively; these pts have shorter follow-up (ie, immature data) with median time on study of 3.1 months. Twenty-nine (66%) of the 44 pts administered ≥40 mg were triple-class refractory and reported an ORR of 55% (16/29).
Conclusions
TNB-383B in pts with RRMM is well tolerated with an ORR of 79% observed at doses ≥40 mg in the dose-escalation cohorts. Despite having a shorter follow-up period, this trend was also observed at doses ≥40 mg in the combined dose-escalation/expansion cohorts (ORR: 64%). Enrollment into the dose-expansion arm is ongoing; updated data will be presented at the meeting.
Kumar: Novartis: Research Funding; Carsgen: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Roche-Genentech: Consultancy, Research Funding; Bluebird Bio: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Beigene: Consultancy; Oncopeptides: Consultancy; Antengene: Consultancy, Honoraria; Tenebio: Research Funding; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding. D'Souza: Imbrium, Pfizer, BMS: Membership on an entity's Board of Directors or advisory committees; Sanofi, Takeda, Teneobio, CAELUM, Prothena: Research Funding; Janssen, Prothena: Consultancy. Shah: Oncopeptides: Consultancy; Nektar: Research Funding; Poseida: Research Funding; Janssen: Research Funding; Indapta Therapeutics: Consultancy; GSK: Consultancy; CareDx: Consultancy; BMS/Celgene: Research Funding; Bluebird Bio: Research Funding; CSL Behring: Consultancy; Teneobio: Research Funding; Kite: Consultancy; Sutro Biopharma: Research Funding; Karyopharm: Consultancy; Amgen: Consultancy; Precision Biosciences: Research Funding; Sanofi: Consultancy. Rodriguez: Takeda: Consultancy, Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Oncopeptides: Consultancy, Honoraria; Amgen: Consultancy, Speakers Bureau. Voorhees: AbbVie Inc, Bristol-Myers Squibb Company; Consulting Agreement: GlaxoSmithKline, Novartis, Oncopeptides: Other: Advisory Committee; Bristol-Myers Squibb Company.: Other: Data Safety & Monitoring. Bueno: AbbVie: Current Employment, Current equity holder in publicly-traded company. Buelow: Teneobio, Inc.: Current Employment, Current holder of stock options in a privately-held company. Freise: Abbvie, Inc.: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Yue: Abbvie, Inc.: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Pothacamury: AbbVie: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Polepally: Abbvie, Inc.: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Vij: BMS: Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; BMS: Honoraria; GSK: Honoraria; Oncopeptides: Honoraria; Karyopharm: Honoraria; CareDx: Honoraria; Legend: Honoraria; Biegene: Honoraria; Adaptive: Honoraria; Harpoon: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal